Precision Oncology - Researching New Treatments Against Triple Negative Breast Cancer
Dr. Matthieu Moncan, 17/09/2024
My name is Matthieu Moncan, I’m a French postdoctoral researcher working with Prof. Adrienne Gorman in the Apoptosis Research Centre at the University of Galway. In 2022, I joined the Precision Oncology Ireland consortium thanks to a MSCA - DevelopMed fellowship to discover new treatments against triple negative breast cancer.
Breast cancer is a disease in which the cells of the breast tissues divide uncontrollably, forming a tumour, before spreading to the other parts of the body and damaging other organs. To prevent this, multiple treatments such as chemotherapy are designed to kill these cells before they can spread.
There are different types of breast cancer. Among them, a specific subtype called “Triple Negative” breast cancer (TNBC) has been shown to be more resistant to treatment. Our research team have discovered that these cells show signs of “cellular stress”. Cellular stress can be defined as any environmental challenge than can cause damages to the cell. Sunlight, smoking and viral infections are common examples of cellular stress inducers. Unfortunately, a stressed cell cannot work properly and can become harmful for other healthy cells, leading to organ damages. Thus, these cells are usually naturally removed from the body through a self-destruct mechanism. However, despite being stressed, TNBC cells are not self-destructing and manage to better resist treatment such as chemotherapy. Our team tries to understand the mechanism behind this paradox.
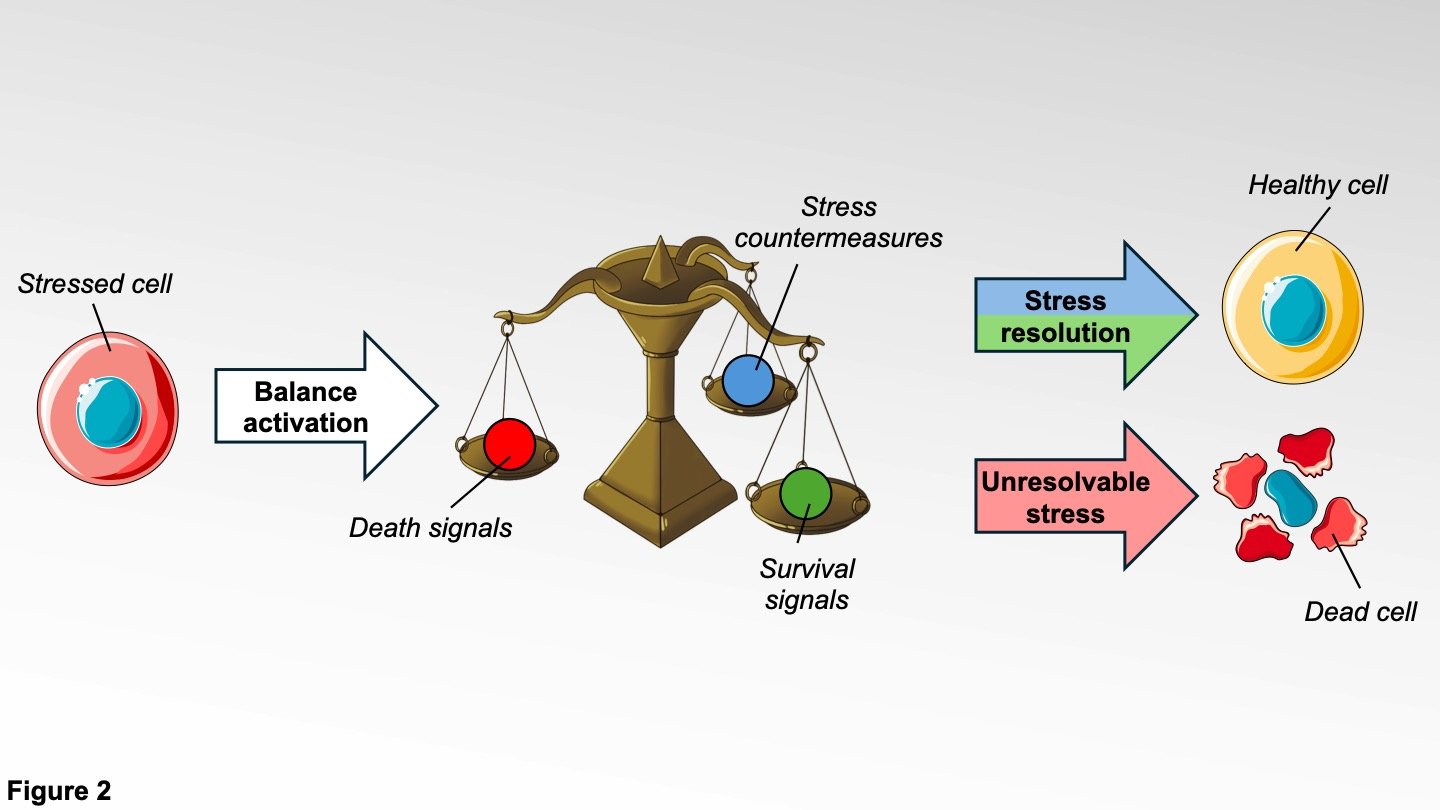
In cells, the management of stress can be viewed as a three arms balance. The first arm activates the stress countermeasures. These countermeasures include every mechanism through which the cell can resolve the origin of the stress. However, these mechanisms need time to be effective. To prevent the cell from self-destructing too early, the second arm activates survival signals that will temporarily keep them alive. If the stress is resolved, the cell is healthy again and can perform its functions. If the stress cannot be resolved, the second arm of the balance will shut down and the third arm will induce death signals, activating the cell self-destruct system.
We have discovered that TNBC cells are able to bypass the natural stress response by keeping the second arm of the balance active, allowing cancer cells to keep expressing survival signals and preventing their elimination. To avoid this, our team is focused on finding new ways to control the stress response balance in order to improve TNBC treatment.
Our research is based on four different objectives. First, we are studying the mechanisms governing the stress response in cancer cells. Although the stress balance itself is well described in the scientific literature, the mechanism through which it induces cell survival or cell destruction are still incompletely understood. Second, we are searching for new inhibitors that could stop the stress countermeasures and the survival signals to block cell division and increase TNBC sensitivity to treatment. Third, we are testing new combinative treatments with previously described chemotherapies and inhibitors of stress pathways to improve cancer cell elimination. And finally, the immune system, the mechanism that helps our body to stay healthy, also has for function to destroy cancer cells. Unfortunately, it has been shown to have difficulties to identify and eliminate stressed cancer cells. Thus, our fourth objective is to understand which mechanisms govern cancer cells resistance to the immune system and find ways to improve their susceptibility to immune cell killing.
Altogether, our goal as a team is to better understand how cancer cells and cellular stress are working, to provide new tools for precision oncology, a new field of research against cancer. Indeed, every cancer, even those coming from the same organ or tissue, are different at the functional and molecular level. This view of cancer has given birth to precision oncology, a field of study based on the analysis of cancer cells’ identity.
Nowadays, many chemotherapy-based treatment are unspecific as they globally target cells which are highly dividing like cancer cells. This unspecific treatment can lead to many side effects which can highly impact the quality of life of patients and does not target specifically the mechanisms at play on the treated cancer.
In the near future, the results of our research and of the whole field of precision oncology, will enable us to analyse each patient’s cancer characteristics. This will permit to better stratify patients depending on their biology and to propose therapeutic strategies which are tailored for them. From there, for patients suffering from cancer subtypes displaying stress features, we propose new therapeutic treatments combining both specific inhibitors of stress mechanisms and use of chemotherapy. By using these inhibitors, we will sensitize cancer to chemotherapy, allowing to reduce the dosage used for treatment. Such strategy will not only improve patient outcome, but also reduce chemotherapy-linked side-effects, improving the patient quality of life.
Dr. Matthieu Moncan
Dr. Matthieu Moncan is a MSCA-funded postdoctoral fellow under the DevelopMed program at the University of Galway. He trained in the European Magisterium in Genetics of Paris University before starting his PhD in the Imagine Institute where he studied the genetic origin of autoimmune diseases and the role of the IRE1α protein in cellular biology and immunity. His current project aims to find new inhibitors which will help regulate specific downstream pathways of IRE1α which are fundamental for cancer progression.
He is representing START at Vetenskap & Allmänhet’s Borrow a Researcher European Edition for #EuropeanResearchersNight! He will talk about cell stress and cancer biology with Swedish school groups on Friday the 27th.
You can follow him on Twitter/X: @MoncanMatthieu
And LinkedIn: Matthieu Moncan | LinkedIn